BEP
Efficient coupling reagent for the synthesis of sterically hindered amides, especially peptides. In terms of reactivity and racemization-suppressing capability, this pyridinium-type reagent, BEP, proved to be more efficient than commonly used uronium- and phosphonium-type reagents, such as HBTU, BOP, and PyBroP, for the synthesis of hindered peptides containing N-methylated or Calpha,Calpha-dialkylated amino acid residues. BEP was also proven to be an efficient reagent for the synthesis of esters, especially active esters and hindered esters, and tert-butyl esters.
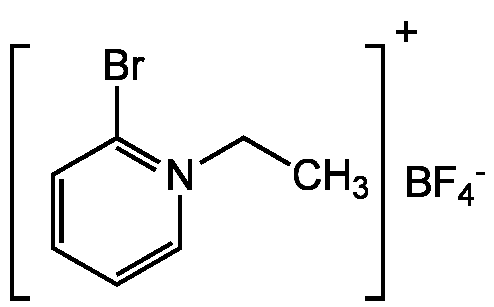
| Catalog Number | CDX-B0040-G001 |
| Alternative Name(s) | 2-Bromo-1-ethyl-pyridinium tetrafluoroborate |
| Research Area | Biochemicals, Immunology |
| Molecular Formula | C7H9BBrF4N |
| CAS# | 878-23-9 |
| Purity | >97% |
| Inchi | InChI=1S/C7H9BrN.BF4/c1-2-9-6-4-3-5-7(9)8;2-1(3,4)5/h3-6H,2H2,1H3;/q+1;-1 |
| Inchi Key | YJDXVQLBIAJTHP-UHFFFAOYSA-N |
| SMILES | [F-].FB(F)F.CC[N]1=C(Br)C=CC=C1 |
| Size | 1 g |
| Supplier Page | http://www.adipogen.com/cdx-b0040/bep.html |

