PTACH
Non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell-permeable prodrug that is intracellularly converted to a potent HDAC inhibitor. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50 = 1.1 – 9.1mM). Also reactivates latent HIV-1 gene expression.
| Catalog Number | CDX-P0063-M005 |
| Alternative Name(s) | NCH 51; Cpd 51; S-[6-(4-Phenyl-2-thiazolylcarbamoyl)hexyl] thioisobutyrate |
| Research Area | Biochemicals, Cancer |
| Molecular Formula | C₂₀H₂₆N₂O₂S₂ |
| CAS# | 848354-66-5 |
| Purity | >95% |
| Inchi | InChI=1S/C20H26N2O2S2/c1-15(2)19(24)25-13-9-4-3-8-12-18(23)22-20-21-17(14-26-20)16-10-6-5-7-11-16/h5-7,10-11,14-15H,3-4,8-9,12-13H2,1-2H3,(H,21,22,23) |
| Inchi Key | MDYDGUOQFUQOGE-UHFFFAOYSA-N |
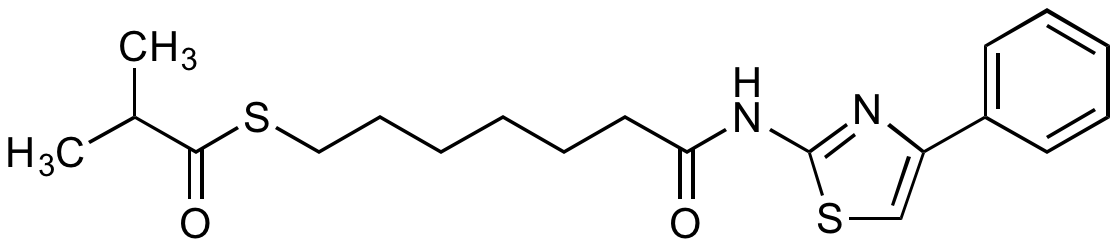
| SMILES | CC(C)C(=O)SCCCCCCC(=O)NC1=NC(=CS1)C1=CC=CC=C1 |
| Size | 5 mg |
| Supplier Page | http://www.adipogen.com/cdx-p0063/ptach.html |

