Shikonin
Anticancer compound. Inhibits TNF-alpha-induced and B-16 melanoma-induced angiogenesis. Induces apoptosis and RIP1- and RIP3-dependent necroptosis in several cancer cells. Circumvents cancer antidrug resistance through the necroptosis pathway. Proteasome inhibitor. Autophagy inducer. Topoisomerase I inhibitor. Inhibits glycolysis in cancer cells by inhibiting tumor-specific pyruvate kinase M2 (PKM2). Anti-inflammatory compound. Inhibits leukocyte migration, downregulates chemokine receptor expression, and inhibits HIV-1 replication. Inhibits the activation of NLRP3 and AIM2 inflammasome. Shown to directly target caspase-1 and as a inhibitor of PKM2 to block PKM2-mediated glycolysis that promotes inflammasome activation by modulating EIF2AK2 phosphorylation in macrophages. Antioxidant. Free radical scavenger. Directly inhibits nitric oxide synthase (NOS). Regulator of systemic glucose tolerance, energy balance and adiposity/obesity. Adipogenesis inhibitor. Shown to inhibit fat accumulation in adipocytes. Antibacterial and antifungal agent.
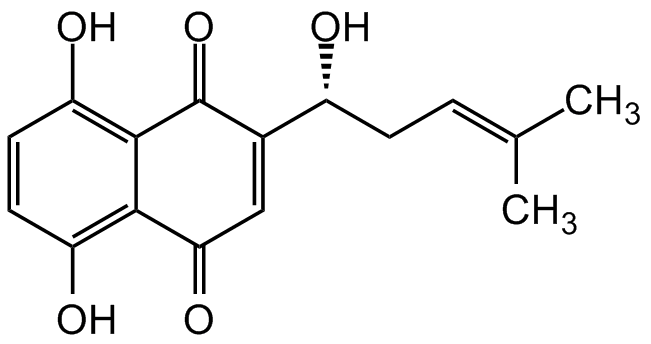
| Catalog Number | AG-CN2-0487-M010 |
| Alternative Name(s) | (+)-Shikonin; (R)-Shikonin; Tokyo violet; NSC 252844; Isoarnebin 4; C.I. 75535; (R)-5,8-Dihydroxy-2-(1-hydroxy-4-methylpent-3-en-1-yl)naphthalene-1,4-dione |
| Research Area | Apoptosis, Biochemicals, Cancer, Cell Death, Immunology, Inflammasomes, Inflammation, Metabolism, Natural Products |
| Molecular Formula | C16H16O5 |
| CAS# | 517-89-5 |
| Purity | >98% |
| Inchi | InChI=1S/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3/t10-/m1/s1 |
| Inchi Key | NEZONWMXZKDMKF-SNVBAGLBSA-N |
| SMILES | OC1=C2C(C(C=C([C@H](O)C/C=C(C)/C)C2=O)=O)=C(O)C=C1 |
| Size | 10 mg |
| Supplier Page | http://www.adipogen.com/ag-cn2-0487/shikonin.html |

