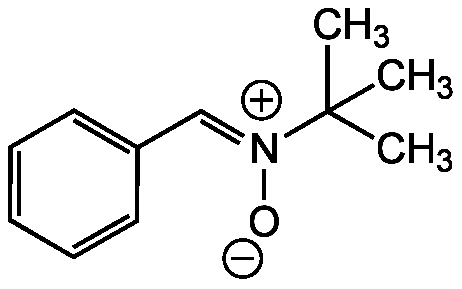
N-tert-Butyl-alpha-phenylnitrone [PBN]
Cell permeable spin trap commonly used in free radical research for both in vivo and in vitro studies. It protects against oxidative damage caused by various inflammatory events, demonstrating neuroprotective, anti-anging, and antidiabetic effects. PBN has been shown to inhibit LPS-induced NF-kappaB DNA binding activity, inhibit COX-2 catalytic activity, inhibit lipid peroxidation in rat liver microsomes and prevent the induction of inducible nitric oxide synthase (iNOS). Shows anti-cancer activity in several experimental cancer models.
| Catalog Number | CDX-B0269-G005 |
| Alternative Name(s) | Phenyl N-t-butylnitrone; N-tert-Butyl-alpha-phenylnitrone; N-Benzylidene-N-(2-methyl-2-propanyl)amine oxide; (Z)-N-Benzylidene-2-methylpropan-2-amine oxide |
| Research Area | Biochemicals, Cancer, Inflammation, Metabolism |
| Molecular Formula | C11H15NO |
| CAS# | 3376-24-7 |
| Purity | >98% |
| Inchi | InChI=1S/C11H15NO/c1-11(2,3)12(13)9-10-7-5-4-6-8-10/h4-9H,1-3H3 |
| Inchi Key | IYSYLWYGCWTJSG-UHFFFAOYSA-N |
| SMILES | CC(C)(C)[N+]([O-])=CC1=CC=CC=C1 |
| Size | 5 g |
| Supplier Page | http://www.adipogen.com/cdx-b0269/n-tert-butyl-alpha-phenylnitrone-pbn.html |

