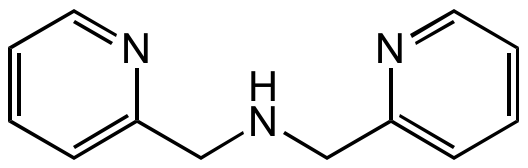
Di-(2-picolyl)amine
Building block for synthesis. DPA is a secondary amine with two picolyl substituents. The compound is a tridentate ligand in coordination chemistry and commonly used to produce Zn-based chemosensors/probes, such as Zinpry. As a tridentate ligand this compound provides three nitrogen donors that affords good selectivity for Zn2+ over biologically relevant metals such as Na+, K+, Mg2+ and Ca2+, and leaves coordination sites free for anion binding. The amino nitrogen of the DPA group is a good candidate as an electron donor in either photoinduced electron transfer or photoinduced charge transfer (PET or PCT) sensors. Zn(II)–DPA complexes are widely used in anion recognition and sensing.
| Catalog Number | CDX-B0134-G005 |
| Alternative Name(s) | Dipicolylamine; DPA; Bis(2-pyridylmethyl)amine; 2,2'-Bis(pyridylmethyl)amine; NSC 176070 |
| Research Area | Biochemicals, Immunology |
| Molecular Formula | C12H13N3 |
| CAS# | 1539-42-0 |
| Purity | >95% |
| Inchi | InChI=1S/C12H13N3/c1-3-7-14-11(5-1)9-13-10-12-6-2-4-8-15-12/h1-8,13H,9-10H2 |
| Inchi Key | KXZQYLBVMZGIKC-UHFFFAOYSA-N |
| SMILES | C(NCC1=CC=CC=N1)C1=CC=CC=N1 |
| Size | 5 g |
| Supplier Page | http://www.adipogen.com/cdx-b0134/di-2-picolyl-amine.html |

