DPM
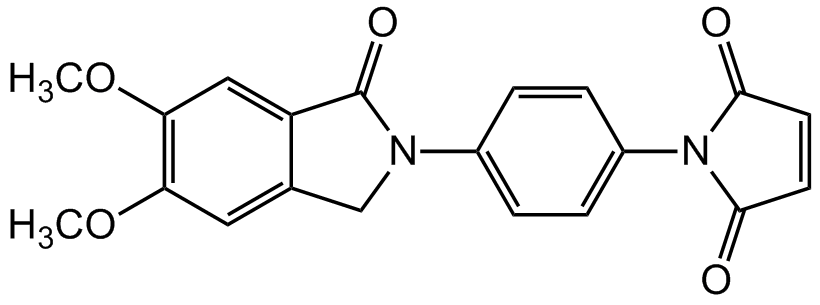
Nonfluorescent N-substituted maleimides react selectively with thiol compounds to form fluorescent derivatives. Fluorescent intensity of the dimethoxyphthalimidyl group is higher than that of the nonsubsituted phthalimidyl group. The analytical sensitivity of N-[4-(5,6-Dimethoxy-N-phthalimidyl)phenyl]maleimide (DPM) is about 3-times higher than that of N-[4-(2-phthalimidyl)phenyl]maleimide (PPM). DPM can be used as a precolumn labeling reagent in HPLC. DPM was also used for the determination of physiologically important thiols in biological fluids or therapeutic drug monitoring.
| Catalog Number | CDX-D0049-M250 |
| Alternative Name(s) | N-[4-(5,6-Dimethoxy-N-phthalimidinyl)phenyl]maleimide |
| Research Area | Biochemicals, Immunology |
| Molecular Formula | C20H16N2O5 |
| CAS# | 143503-03-1 |
| Purity | >97% |
| Inchi | InChI=1S/C20H16N2O5/c1-26-16-9-12-11-21(20(25)15(12)10-17(16)27-2)13-3-5-14(6-4-13)22-18(23)7-8-19(22)24/h3-10H,11H2,1-2H3 |
| Inchi Key | ISCHQGBNSNVWCQ-UHFFFAOYSA-N |
| SMILES | COC1=C(OC)C=C2C(CN(C2=O)C2=CC=C(C=C2)N2C(=O)C=CC2=O)=C1 |
| Size | 250 mg |
| Supplier Page | http://www.adipogen.com/cdx-d0049/dpm.html |

