Gentamicin sulfate (USP Grade)
Aminoglycoside antibiotic. Protein synthesis inhibitor. Causes codon misreading by binding to the 30S ribosomal subunit, blocking the translocation of peptidyl-tRNA from the acceptor site to the donor site. Antibacterial against Gram-negative aerobic bacteria, Gram-positive bacteria and mycoplasmas. Used as a selection agent (gentamicin-resistance gene) in molecular biology applications. Broad-spectrum cell culture antibiotic that is nontoxic to viruses and mammalian cells at antibacterial and antimycoplasmal concentrations. Due to its extended stability and slow development of bacterial resistance, it is a useful antibiotic in long-term virus und tissue culture studies. Bactericidal effects are exerted by the binding to the outer membrane, causing disruption of the membrane. This increases the permeability of the cell envelope, leakage of cell contents, and leading to apoptosis and proteolysis (cell death). Causes also cell death by generation of free radicals, phospholipidosis, extracellular calcium-sensing receptor stimulation and energetic catastrophe.
| Catalog Number | AG-CN2-0066-G025 |
| Alternative Name(s) | Apogen; Garamycin; Gentiomycin C; Refobacin; NSC-82261 |
| Research Area | Antibiotic, Apoptosis, Cell Death, Inflammation, Natural Products |
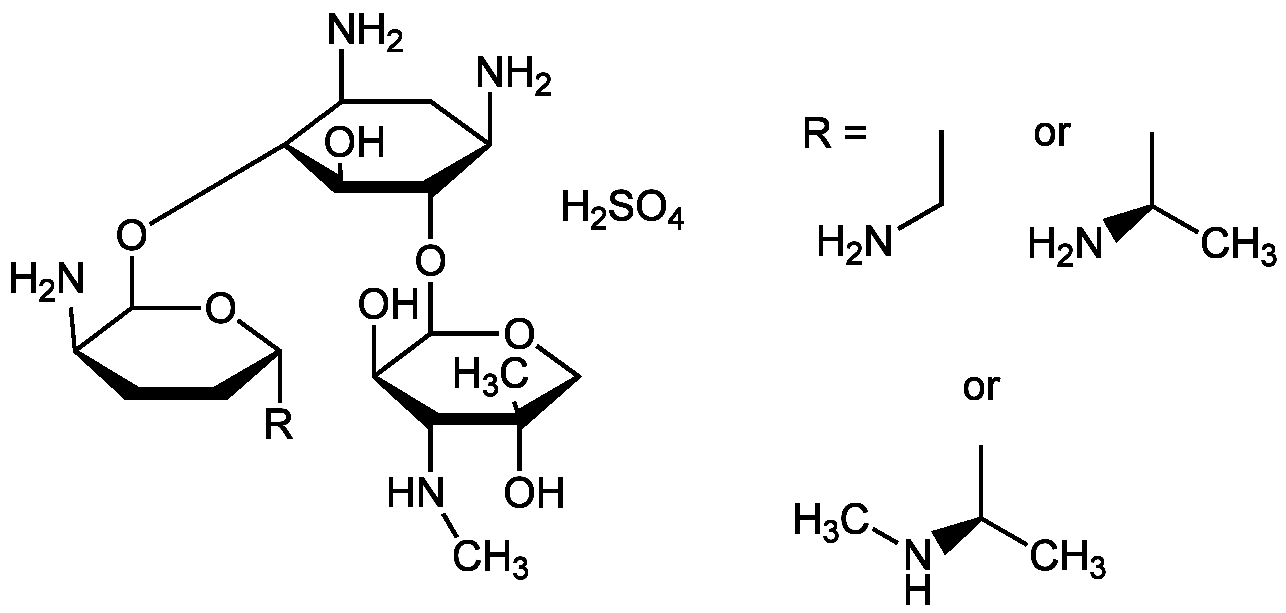
| Molecular Formula | C21H43N5O7 . H2SO4 (unspecified) |
| CAS# | 1405-41-0 |
| Inchi | InChI=1S/C19H39N5O7.H2O4S/c1-19(27)7-28-18(13(26)16(19)24-2)31-15-11(23)5-10(22)14(12(15)25)30-17-9(21)4-3-8(6-20)29-17;1-5(2,3)4/h8-18,24-27H,3-7,20-23H2,1-2H3;(H2,1,2,3,4)/t8-,9+,10-,11+,12-,13+,14+,15-,16+,17+,18+,19-;/m0./s1 |
| Inchi Key | HNCAOLPMSASREN-UCMBPTNBSA-N |
| SMILES | CCN.CC(C)N.CNC(C)C.OS(O)(=O)=O.CN[C@H]1[C@@H](O)C(OC2[C@H](N)CC(N)[C@H](OC3O[C@H]([*])CC[C@H]3N)[C@H]2O)OCC1(C)O |
| Size | 25 g |
| Supplier Page | http://www.adipogen.com/ag-cn2-0066/gentamicin-sulfate-usp-grade.html |

